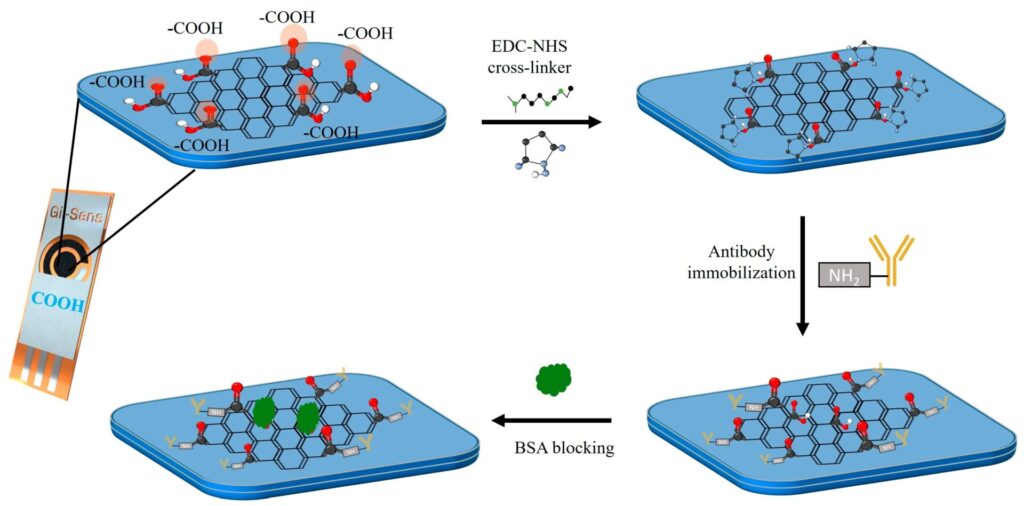
Polymer of intrinsic microporosity (PIM-1) enhances hydrogen peroxide (H2O2) production at Gii-Sens electrodes
Highlights
- The goal of this paper was to catalyse a reaction whereby the polymer coating absorbs oxygen to enhance hydrogen peroxide production using Gii-Sens.
- Graphene and graphene-oxide-related materials have been shown to be active in producing hydrogen peroxide cathodically but the yield of H2O2 may vary, dependent on conditions, defects, or doping.
- Here, it is demonstrated that the formation of H2O2 on graphene foam can be enhanced by microporous polymer deposits on the Gii electrode (Gii-Sens) surface.
Abstract
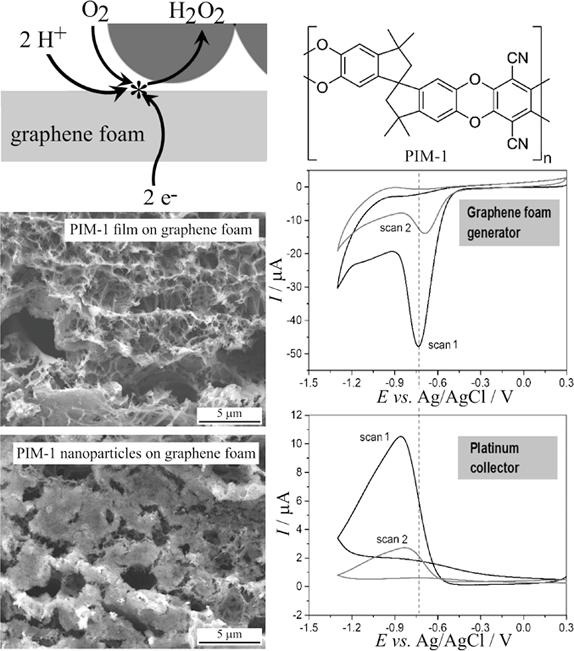
3D-graphene foam electrodes (Gii-Sens) immersed in a phosphate buffer solution of pH 7 are shown to generate hydrogen peroxide at a significantly faster rate in the presence of a nanoparticulate polymer of intrinsic microporosity (PIM-1). The effect is demonstrated to be associated at least in part with oxygen binding into PIM-1 under triphasic conditions. The release of the oxygen at the electrode|solution interface quadruples H2O2 production. Generator–collector experiments are performed with a graphene foam disk generator and a platinum disk electrode collector to allow in situ detection of hydrogen peroxide and oxygen.
Introduction
Polymers of intrinsic microporosity (PIMs) are molecularly rigid and contorted structures which are unable to pack and are therefore able to form films of high microporosity [1] with a typical 1000 m2 g−1 surface area and typical 1 nm pore size. PIMs have found applications primarily in gas phase separation and gas binding [2], but recently have also started to attract interest in electrochemistry [3]. Interfacial redox processes that have been investigated include the CO2 reduction [4], formic acid oxidation [5], the Fe(CN)63-/4- system [6], bound quinones [7], and the oxygen reduction reactions [8].
For the oxygen reduction reaction, the onset for formation of hydrogen peroxide was noted to be shifted to positive potentials in the presence of PIM-1 (see molecular structure in Fig. 1), indicative of a locally higher oxygen activity under triphasic conditions (gas|liquid|polymer).
Electrode processes involving redox-active gases dissolved in aqueous media have been shown to be beneficially affected by the presence of polymer of intrinsic microporosity coatings on electrodes, presumably via adsorption of gas into the wet microporous structure [9].
More generally, it has recently been highlighted by Erdosy et al. [10] that a wider range of materials with intrinsic microporosity physically increase the apparent solubility of poorly water-soluble gases like oxygen under triphasic conditions.